About Clinical Research
Through pioneering clinical trials at the Research Institute of the Southeast, Dr. Kenneth Beer and his team have led the advancement of dermatologic research for more than a decade. At our West Palm Beach and Jupiter locations, patients are given the opportunity to participate in studies that assist fellow doctors advance their techniques, while helping themselves and others overcome their skin issues.
Today, the Research Institute of the Southeast is regarded as one of the top clinical research sites in the nation, and has revolutionized treatment standards in general and aesthetic dermatology. Our full-time dedicated research staff and state-of-the-art facilities enable efficient and timely clinical trial completion with full FDA compliance.
The Research Institute of the Southeast is currently conducting Phase II through Phase IV clinical research studies.
Clinical Trials by Research Institute of Southeast by Beer Dermatology

What is a Clinical Trial?
A clinical trial, also called clinical research is a research study in human volunteers to answer specific health questions. Carefully conducted clinical trials are the fastest and safest way to find treatments that work in people, in ways to improve health.
Interventional Trials VS Observational Trials
Interventional trials determine whether experimental treatments or new ways of using known therapies are safe and effective under controlled environments.
Observational trials address health issues in large groups of people or populations in natural settings.
Why participate in a clinical trial?
Participants in clinical trials can play a more active role in their own health care, gain access to new research treatments before they are widely available and help others by contributing to medical research.
All of the clinical trials are done at no cost to the subjects. You may even be compensated for your time and travel if you qualify and participate.
Who can participate in a clinical trial?
All clinical trials have guidelines about who can participate. Using inclusion and exclusion criteria is an important principle of medical research that helps to produce reliable results.
The criteria are used to identify appropriate participants and keep them safe. The criteria help ensure that researchers will be able to answer the questions they plan to study.
Can a participant leave a clinical trial after it has begun?
Yes, a participant can leave a clinical trial at any time. When withdrawing from the trial the participant should let the research team know about it and the reasons for leaving the study.

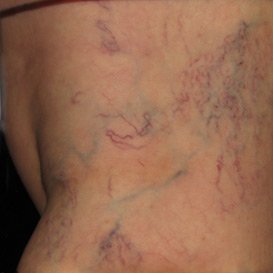
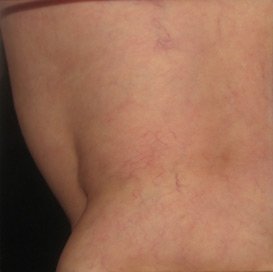
 Sclerotherapy can eliminate painful and unsightly side effects of vein disease. The injectable improves both comfort and appearance quickly and effectively, often in only 2-4 treatments.
Sclerotherapy can eliminate painful and unsightly side effects of vein disease. The injectable improves both comfort and appearance quickly and effectively, often in only 2-4 treatments.
 Radiesse is an injectable that restores lost volume, eliminating unwanted facial wrinkles and folds that commonly occur with age. One treatment produces almost immediate results.
Radiesse is an injectable that restores lost volume, eliminating unwanted facial wrinkles and folds that commonly occur with age. One treatment produces almost immediate results. 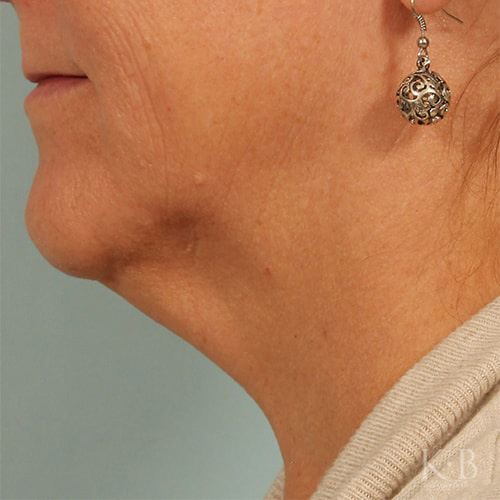
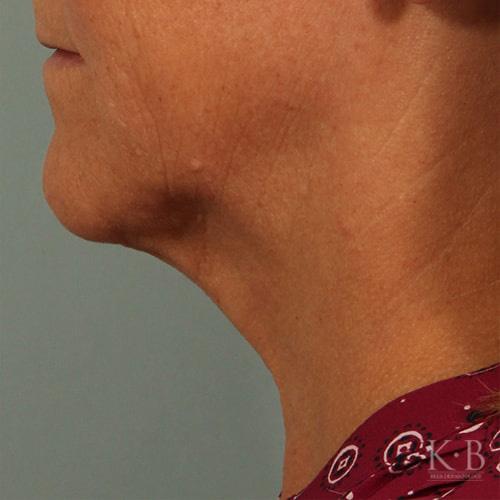
 Kybella is an FDA-approved injectable to eliminate submental fat, also known as a double chin. The injection permanently destroys fat cells for a sleek, slimmer facial contour.
Kybella is an FDA-approved injectable to eliminate submental fat, also known as a double chin. The injection permanently destroys fat cells for a sleek, slimmer facial contour.
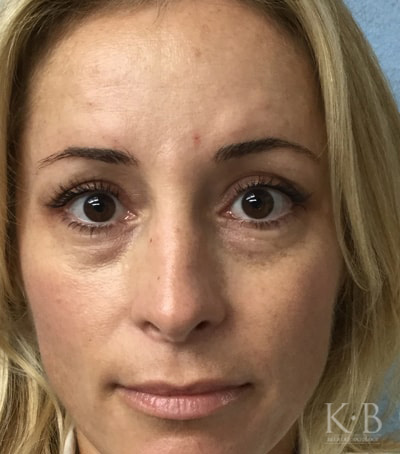
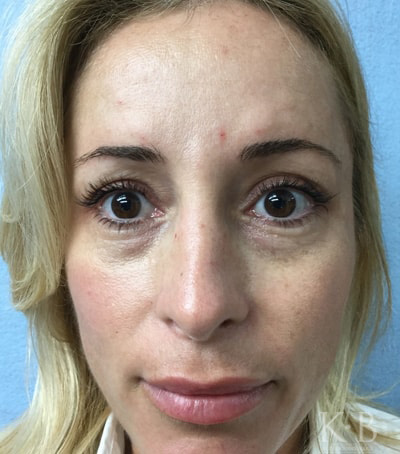
 Restylane is an injectable filler that can fill out wrinkles, volume-loss, and deep lines in the face. It can be used to address crow’s feet, nasolabial folds, and marionette lines.
Restylane is an injectable filler that can fill out wrinkles, volume-loss, and deep lines in the face. It can be used to address crow’s feet, nasolabial folds, and marionette lines.
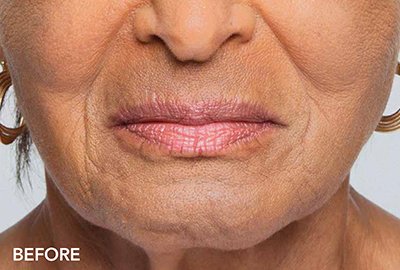
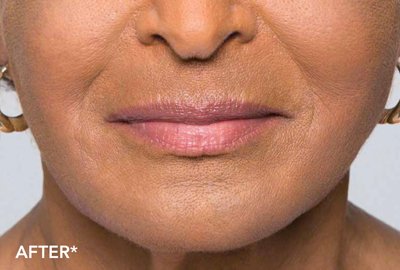
 Belotero is a type of injectable filler that will soften lines and wrinkles around the mouth. The filler integrates with your own skin tissue for results that are natural looking.
Belotero is a type of injectable filler that will soften lines and wrinkles around the mouth. The filler integrates with your own skin tissue for results that are natural looking.


 Scultpra stimulates collagen production to gradually restore and improve losses in facial volume as a result of aging. Results can last up to two years.
Scultpra stimulates collagen production to gradually restore and improve losses in facial volume as a result of aging. Results can last up to two years.
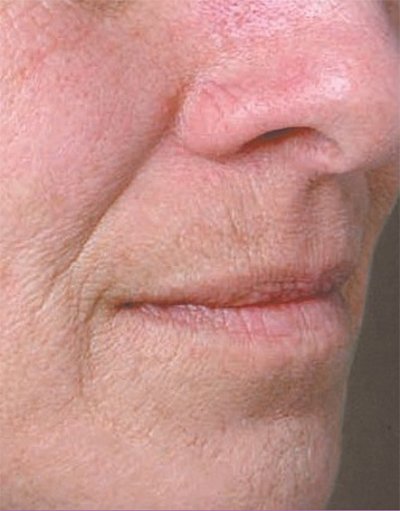
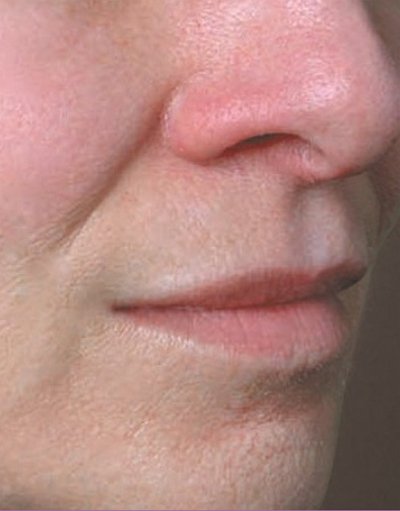
 Juvederm Volbella is specially designed to add volume to the lips, and minimize lines that form around the month. The result is fuller, more youthful looking lips.
Juvederm Volbella is specially designed to add volume to the lips, and minimize lines that form around the month. The result is fuller, more youthful looking lips.

 IPL photofacial, or photorejuvenation, evens out facial color, removes red spots from the face and can reduce the visibility of acne, rosacea, and other common skin problems.
IPL photofacial, or photorejuvenation, evens out facial color, removes red spots from the face and can reduce the visibility of acne, rosacea, and other common skin problems.


 Botox injectable fillers temporarily softens dynamic wrinkles in the upper half of the face that are a Results often last for up to 6 months, after which maintenance sessions are needed.
Botox injectable fillers temporarily softens dynamic wrinkles in the upper half of the face that are a Results often last for up to 6 months, after which maintenance sessions are needed. 






